Although primary resonance stabilized carbocations allyl cation benzyl cation and methoxymethyl cation are less stable than tertiary carbocations secondary resonance stabilized carbocations more stable than tertiary carbocations.
Which is more stable vinyl carbocation or a primary carbocation.
We have one more case in this example with primary carbocations 1 and 5.
It is possible to demonstrate in the laboratory see section 16 1d that carbocation a below is more stable than carbocation b even though a is a primary carbocation and b is secondary.
Carbon with two other atoms attached prefers sp hybridization and a linear geometry.
Some professors will rank a primary benzylic carbocation under or near a tertiary carbocation.
Vinyl cations are located on sp hybridized carbons.
Carbon of the double bond and this is the least stable.
In general you probably won t see a primary or methyl carbocation in o chem 1.
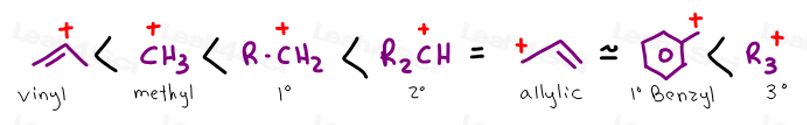
Primary phenyl vinyl methyl allyl benzyl methoxymethyl most stable least stable important note.
The hybridization of a vinyl carbocation is sp hybirdized.
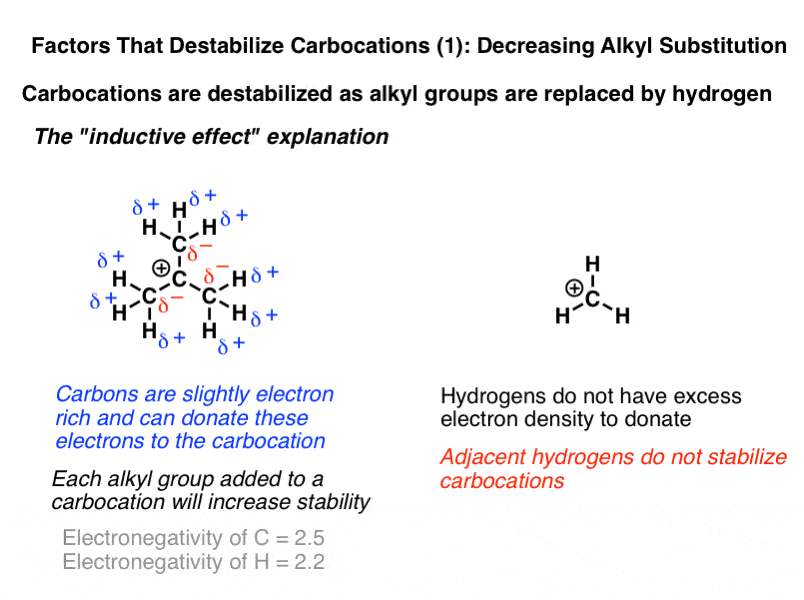
The more r groups a carbocation has attached the more stable it is.
But carbocation 5 is vinylic carbocation positively charged carbon is sp 2 hybridized i.
This shares the burden of charge over 4 different atoms making it the most stable carbocation.
As you increase substitution the benzylic carbocation becomes more and more stable.
Compound i is more stable among all as it is a tertiary carbocation then comes compound ii which is a secondary carbocation compound iv is a primary carbocation compound ii is a secondary carbocation with an electron withdrawing group close to it which makes it unstable.
The most stable version is the tertiary benzylic carbocation.
The vinyl cations are less stable due to the difference in hybridization of the carbon bearing the positive charge.
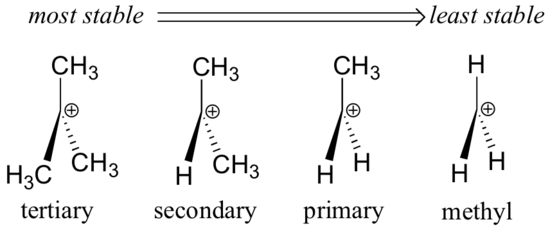
That means that tertiary is more stable than secondary secondary more stable than primary and primary more stable than methyl.